Major royal jelly proteins of the honeybee Apis mellifera.
Major royal jelly proteins (MRJPs) have been first identified in royal jelly (RJ), a secretion of the hypopharyngeal glands of young worker bees that is used to rear female larvae to majestic queens (Snodgrass, 1925). Due to the high abundance of essential amino acids in these proteins it has been assumed that they have mainly nutritional functions during larval development (Schmitzová et al., 1998). With the release of the honeybee genome (Honeybee Genome Sequencing Consortium, 2006) it became clear that MRJPs are not only comprised of the four MRJPs actually present in RJ but moreover of nine proteins whose encoding genes (mrjp1 – 9) are located in a tandem array on chromosome 11 flanked by the genes yellow-e3 and yellow-h.
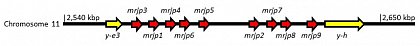
Screening currently available genomes, we found that mrjps are common in Hymenopteran species but mostly restricted to one copy also located between the two yellow genes (Buttstedt et al., 2014). In addition to the honeybee, cases of copy number radiations arose in some Hymenopteran species including the leaf cutter ant Atta cephalotes and the parasitic wasp Nasonia vitripennis. However, mrjps have up to now never been found outside of the Hymenoptera and thus they seem to be limited to the order but are not a prerequisite for survival as some species, e.g., the fire ant Solenopsis invicta, can perfectly live without.
With our current research two important questions are approached:
1.) What are the functions of these major royal jelly proteins?
2.) Why does the honeybee need so many of them?
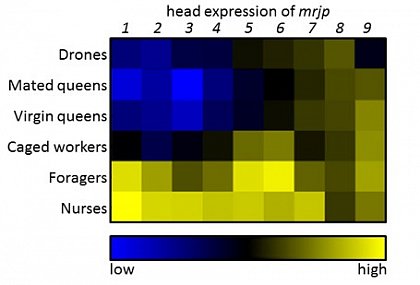
In a first study we determined the expression of the nine mrjps in various body parts (head, thorax, abdomen) in all honeybee castes and both sexes (Buttstedt et al., 2013). We found that mrjp8 and 9 have been evenly expressed among the body parts whereas the expression of mrjp1 to 7 was focused in the worker heads. Interestingly, expression has not only been up-regulated in brood rearing nurses with active food glands but also in foraging bees compared to the caged controls. Thus, the functions of MRJPs are clearly not limited to the food glands.
Currently, we are aiming on the functions of the proteins in different complementing ways:
(1) Gene evolution of mrjps
The substitution rates of coding and non-coding nucleotides of all mrjps among Apis species and A. mellifera subspecies will be analysed.
(2) Protein biochemical analyses of MRJPs
The different MRJPs purified from RJ or recombinantly produced will be biophysically characterized (in cooperation with Prof. Dr. Markus Pietzsch).
(3) Impact of MRJPs on honeybee larvae
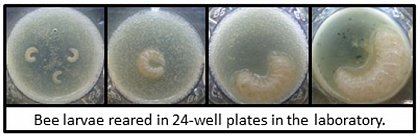
The effects of the MRJPs will be tested on developing larvae during in vitro rearing experiments.
Funding:
11/2014 – current:
Deutsche Forschungsgemeinschaft (DFG) – grant MO 373/32-1 to RFAM
07/2013 – 06/2014:
Fellowship of the Prorectorate for Research and Young Academics of the Martin-Luther- University Halle-Wittenberg to AB
Publications:
Vezeteu TV, Bobiş O, Moritz RFA, Buttstedt A (2016): Food to some, poison to others - honeybee royal jelly and its growth inhibiting effect on European Foulbrood bacteria. MicrobiologyOpen early online
Buttstedt A, Ihling CH, Pietzsch M, Moritz RFA (2016): Royalactin is not a royal making of a queen. Nature 537(7621): E10-E12.
Buttstedt A, Moritz RFA, Erler S (2014): Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol Rev 89(2):255-269 .
Buttstedt A, Moritz RFA, Erler S (2013): More than royal food - Major royal jelly protein genes in sexuals and workers of the honeybee Apis mellifera. Front Zool 10: 72 . Highly accessed.



